Sanofi and Regeneron File Respondents’ Brief on Amgen v. Sanofi | McDonnell Boehnen Hulbert & Berghoff LLP
Sanofi and Regeneron filed their brief at the Supreme Court in Amgen v. Sanofi, in which Amgen seeks to have the Court overturn the District Court’s grant of JMOL in the issue of whether Amgen’s claims were invalid for non-enablement under 35 U.S.C. § 112(a) (see “Amgen Files Its Principal Brief in Amgen v. Sanofi”).
To recap, the Question Presented on which the Supreme Court granted certiorari is:
Whether enablement is governed by the statutory requirement that the specification teach those skilled in the art to “make and use” the invention, 35 U.S.C. § 112, or whether it must instead enable those skilled in the art “to reach the full scope of claimed embodiments” without undue experimentation ― i.e., to cumulatively identify and make all or nearly all embodiments of the invention without substantial “time and effort,” Pet. App. 14a [emphasis in Question].
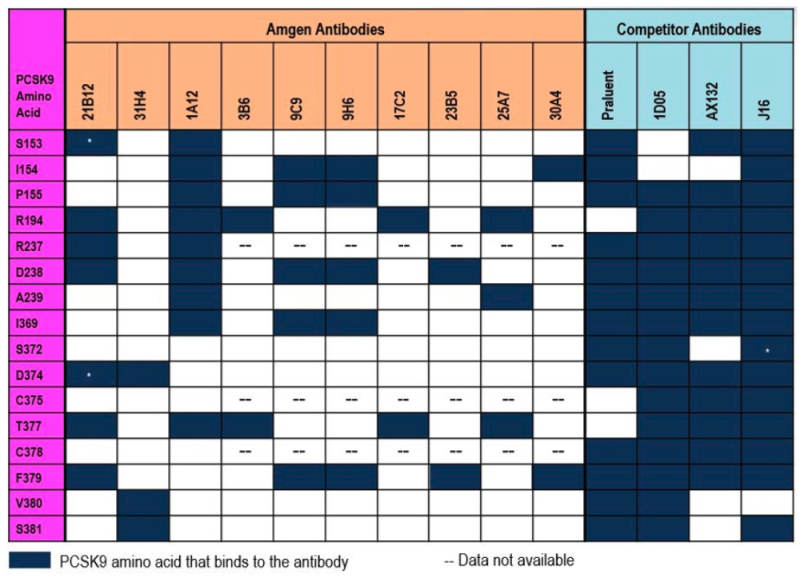
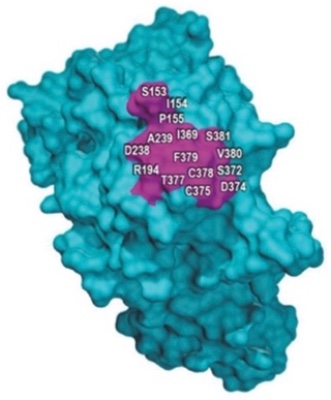
The claims at issue in Amgen’s asserted patents, U.S. Patent Nos. 8,829,165 (“‘165 patent”) and 8,859,741 (“‘741 patent”), encompassed antibodies that recognized specific amino acids in the amino acid sequence of the PCSK9 protein, reciting no structural features or limitations of these antibodies but rather the combination of “target” amino acids, as illustrated in Amgen’s brief:
The Federal Circuit upheld the District Court’s grant of JMOL for non-enablement based on the lack of disclosure regarding the structure of antibodies falling within the scope of the claim in the face of what the District Court understood to be the large number of antibodies (the patents “cover . . . a vast scope of possible antibodies,” potentially “millions,” or “millions and millions” and implicate “an astronomically large number” of candidates) having the claimed binding properties and the small number (26) of antibodies disclosed in Amgen’s specification, as well as the “randomness in how the immune system” produces antibodies means that each immunization creates a “big pool of potential [antibodies],” wherein “you don’t know what you’re going to get,” characterizing such efforts as being “a fishing expedition” (a characterization that may resonate with the Supreme Court).
Sanofi’s brief begins with an appropriate amount of advocacy, admirably avoiding purple prose in describing their response to Amgen’s assertions in its Opening Brief, stating that Amgen’s filing of the patent at issue was strategically aimed at preventing Sanofi from marketing its PCSK9 antibody Praluent® (alirocumab) “in a blatant attempt to corner the market” by claiming a genus of antibodies by function rather than structure (noting that its patent claimed its Praluent antibody by its amino acid structure), and then “asserting a monopoly over an entire genus of functionally defined claims.” In so doing, Sanofi argues Amgen created the “high hurdles” Amgen accuses the Federal Circuit of raising by attempting to contravene the “commonsense proposition” that “the more companies claim as their patent monopoly, the more they must enable” which is “the heart of the patent bargain.” Regarding the disclosure Amgen relies upon in ts specification, the brief asserts that it merely tells the skilled artisan “to make claimed antibodies by randomly generating and testing candidates via processes well-established in the prior art.” The brief illustrates the scope of Amgen’s claims with regard to four antibodies made by Amgen’s competitors (including Praluent) and how different they were from the antibodies actually disclosed in Amgen’s specification:
Regarding the policy consequences attendant upon Amgen’s position the brief states that:
The simple reality is that Amgen has claimed a monopoly over far more than it has enabled. Such claims are not just invalid, but dangerous. They can take medicines away from physicians and patients and could allow someone without a clinically valid species to claim an entire genus of medically-vital antibodies they have not yet discovered.
The brief also emphasizes the health risks of LDL-C and attendant importance of Praluent in addressing this risk (which Amgen’s patent threatens) and that the matter before the Court does not involve Amgen’s patent on Repatha® itself, countering Amgen’s arguments in its brief that it had invested “billions of dollars and a decade of research bringing [it] to market” as a reason to overturn the Federal Circuit’s decision.
On the law Sanofi argues that the Federal Circuit’s straightforward application of the enablement standard applying the Wands factors was entirely consistent with the history of the statute and judicial interpretations thereof, including importantly Supreme Court precedent. This standard “has long been understood to require sufficient disclosure to enable a skilled artisan to make the entire invention claimed, not just a subset, without the need for any significant independent experimentation” under that precedent. The brief characterizes as a “straw man” Amgen’s argument in its Opening Brief that the Federal Circuit has established a new, unnecessarily stringent test for enablement.*
The Argument section of the brief assays the precedential history of the Supreme Court’s and lower courts’ upholding the conventional enablement standard and its relationship to the patent bargain, wherein the scope of enabled claims is determined and cabined by what is disclosed, citing Pennock v. Dialogue, 27 U.S.1, 23 (1829) (Story, J.); J.E.M. Ag Supply, Inc. v. Pioneer Hi-Bred Int’l, Inc., 534 U.S. 124, 142 (2001); Festo Corp. v. Shoketsu Kinzoku Kogyo Kabushiki Co., 535 U.S. 722, 736 (2002), and precedents under English law, King v. Arkwright, (K.B. 1785). The brief draws parallels regarding this understanding of what is required to satisfy enablement under English law to enactment of the first Patent Act in 1790 and the consistency of this standard in subsequent patent law enactments. This interpretation of the enablement statute is supported in the brief by a thorough explication of judicial precedent consistent with Sanofi’s argument that scope requiring undue experimentation has historically been deemed inadequate, including Consol. Elec. Light Co. v. McKeesport Light Co. (The Incandescent Lamp Patent), 159 U.S. 465, 474-75 (1895) (“painstaking experimentation”; Holland Furniture Co. v. Perkins Glue Co., 277 U.S. 245, 256-57 (1928) (“elaborate experimentation”), and finally the modern “undue experimentation” standard adopted by the CCPA in In re Folkers, 344 F.2d 970, 976 (C.C.P.A. 1965), and adopted by the Federal Circuit in In re Wands, 858 F.2d 731 (Fed. Cir. 1988) (reciting the Wands factors).
The brief then sets forth the recent history of cases illustrating how the Federal Circuit (consistent with this precedent) has held cases to be non-enabled “if a claim encompasses ‘thousands’ of ‘candidate compounds,’ and ‘testing’ or ‘screening’ of each candidate is necessary ‘to determine which . . . meet [the] claim,'” citing Idenix Pharms. LLC v. Gilead Scis. Inc., 941 F.3d 1149, 1156-58, 1162-63 (Fed. Cir. 2019); Enzo Life Scis., Inc. v. Roche Molecular Sys., Inc., 928 F.3d 1340, 1345-49 (Fed. Cir. 2019); Wyeth & Cordis Corp. v. Abbott Labs., 720 F.3d 1380, 1384-86 (Fed. Cir. 2013), characterizing these decisions as “guard[ing] against what Amgen has done here: claim a lot and disclose a little, such that skilled artisans (and even Amgen’s own scientists) must engage in undue experimentation because the disclosure leaves them guessing as to how to make and use the full scope of the claimed invention.”** This is not a “special rule” for functional genus claims, the brief argues but rather a consequence of “some genus claims assert a monopoly over far more than they enable,” contrary to both the statute and precedent.
Turning to a direct confrontation of Amgen’s arguments in its Opening Brief, Sanofi contends that Amgen has provided the Court with no persuasive reasoning for changing the established standard (under which Sanofi contends the District Court and Federal Circuit properly invalidated Amgen’s claims on enablement grounds). As characterized by the brief, Amgen itself has not challenged the conventional test (or otherwise would have had to argue contrary to its own arguments below). Rather, the brief argues, Amgen has created, by a “profound mischaracterization” of the decisions below, a chimerical “new” and “different” enablement standard, the “full-scope” requirement, that Sanofi argues is not what the Federal Circuit held, citing language from that Court’s decision expressly disclaiming such a standard (the decision “specifically resisted what might be termed a simple ‘numerosity’ or ‘exhaustion’ requirement,” and that any assertion that the Federal Circuit had “adopted a ‘numbers-based standard’ to evaluate enablement” that asked “how long it would take to make and screen every species” simply “mischaracterizes our law”). In the Federal Circuit’s view (as explained in the brief) “[t]he problem was that the unpredictability of the science combined with a broad functional claim that ‘extend[ed] far beyond the examples and guidance provided’ left skilled artisans with no ability to generate specific undisclosed embodiments and no option but to engage in trial and error using well-established techniques to generate candidate antibodies that would then still need to be tested to ascertain whether they came within the claimed genus.” And Amgen nowhere has identified language in the Federal Circuit’s opinion reciting its “new standard” according to the brief. The important distinction made in the brief between what the Federal Circuit held and what Amgen asserts its decision means is:
When a specification enables a skilled artisan to predictably make specific undisclosed embodiments under circumstances where making all of them would take time, the cumulative effort needed to make and use every single embodiment of the claimed invention is unproblematic. But when the specification provides no useful guidance to skilled artisans to make and use specific undisclosed embodiments and consigns them to a trial-and-error process to unpredictably generate antibodies that must then be tested to determine whether they even fall within the broad functionally claimed genus, Federal Circuit precedent along with this Court’s precedent and statutory text all indicate that the claimed invention is not enabled.
This does not constitute a new test according to the brief, contrasting the situation with the patents before the Court with Amgen’s and Sanofi’s patents on their Repatha and Praluent antibodies, respectively, which provide an unchallenged enabling disclosure of the amino acid sequence of these antibodies.
The brief also argues that the Court should reject Amgen’s “as-needed” enablement test advocated as an alternative, based on its inconsistency with the “statutory text, settled precedent, or longstanding practice,” providing extensive analysis of precedent and the history of enablement law in support of its position.
The brief then sets forth Sanofi’s arguments that, contrary to Amgen’s assertions it is Amgen’s position(s) that threaten to harm innovation, by permitting Amgen (and applicants in Amgen’s position in future) to patent protection for claims outside what has been disclosed and thus precluding others from their inventions that have been enabled. Under current Federal Circuit precedent, innovation has not been harmed according to the brief (disregarding the one non-precedential decision by the PTAB cited in Amgen’s Opening Brief, which was based on conventional application of the Wands factors). The brief also cites several instances where the Federal Circuit and district courts have upheld satisfaction of the enablement requirement in biotech and pharma cases, specifically Bayer Healthcare LLC v. Baxalta Inc., 989 F.3d 964, 970-71, 980-81 (Fed. Cir. 2021), and Erfindergemeinschaft UroPep GbR v. Eli Lilly & Co., 276 F.Supp.3d 629, 659-663 (E.D. Tex. 2017), and noting that innovation in these areas has proceeded without enablement impediment in these industries (citing paradoxically Prof. Karshtedt’s law review article in support of this argument). The brief cites the doctrine of equivalents to counter Amgen’s arguments against the threat of mere copyists if genus claims of broad scope are precluded by the enablement requirement, and the countervailing risk to innovation Sanofi asserts is raised by claims having Amgen’s scope. The brief argues that this case “perfectly illustrates the risks” wherein Amgen’s patents if upheld would ban Sanofi’s Praluent from the marketplace and prevent patients needing it from getting it, particularly to the extent that Praluent is available in dosages Amgen cannot provide (“allowing a company that has discovered only particular species to obtain a patent on a broad functionally claimed genus creates a very real risk that important medical treatments will never reach the market”). The brief uses the history of Pfizer’s unsuccessful attempts to gain regulatory approval for its anti-PCSK9 antibody as a “cautionary tale,” where if Pfizer had adopted the claiming strategy followed by Amgen “it would have stifled innovation, and patients would have had no PCSK9 antibody therapies at all—or, at best, would have had to wait longer for Pfizer to develop a safe and effective antibody through trial and error.”
Finally, Sanofi’s brief sets forth specifically why Amgen’s specification is not enabling for the scope of the functionally defined genus claims at issue before the Court, why Sanofi agrees that the case “does not require a third trip through the Federal Circuit” and that the decisions below should be affirmed.
There are a plethora of amicus briefs on both sides of the issue in this case, and those briefs will be the subject of future posts. The Supreme Court should render its decision by the end of the current term.
* In this regard the brief distinguishes Professor Dmitry Karshtedt’s law review article on “The Death of the Genus Claim” by stating even those authorities ultimately concede that innovation has not been harmed by the decision.
** What these cases arguably do is remove the conventional application of the Wands standard that extensive experimentation is not undue if it is routine such that the extensiveness of the experimentation can itself be undue and thus non-enabled (despite the Federal Circuit’s assertions to the contrary).