Recent Developments and Future Perspectives
“In the realm of intellectual property, it is notable that the number of published patent applications has increased considerably since 2019, reflecting a greater investment in cannabis-related technologies in recent years.”
It is not news that cannabis-based medicines have been used for millennia around the world in the treatment of conditions and diseases such as chronic pain; epilepsy; neuropsychiatric disorders; nausea and vomiting related to chemotherapy; symptoms associated with autistic spectrum disorder; amyotrophic lateral sclerosis; rheumatoid arthritis, among others. However, this topic has been the subject of recent discussions due to the progressive dissemination of scientific data proving the efficacy and safety of their uses. New scientific research and clinical studies are expected, not to mention the revision/alteration of legislation in some countries so that the cultivation, production, commercialization and use of cannabis-based medicines becomes more viable and comprehensive.
In Brazil, the sale and production of cannabis-based products is allowed, but pharmaceutical companies are obliged to import the active ingredients used in formulations, which ends up making the medicine available on the market more expensive and difficult for patients to access the treatment. In view of the high cost of cannabis derivatives, Brazilian patients have sought to enforce their right to cultivate the plant before the courts.
Brazil is among those countries currently reviewing legislation to make access to cannabis-based medicines more viable for the population. The first quarter of this year was marked by technical and legal developments, as summarized below, which certainly bode well for access to cannabis and its derivatives in the near future:
- On January 31, 2023, the governor of São Paulo State sanctioned a law that guarantees the free supply of cannabis-based medicines by the Unified Health System (SUS) in the State. Before this law, due to the high cost, cannabis-based medicines were only provided by the SUS as a result of affirmative court decisions, which made the process very slow and bureaucratic. From a national perspective, Senator Paulo Paim created a federal bill to regulate the supply of cannabis-based products in the SUS.
- In March/2023, the Superior Court of Justice (STJ) found that it had the prerogative to decide on the cultivation of cannabis in Brazil, opening the possibility for the legal cultivation of the plant for medicinal and industrial purposes after the subject was halted in the Congress for years. In view of this, a final decision on the merits may be announced by the STJ within a year, which would be a landmark in terms of dealing with an issue that has long been blocked by the conservative contingent in the Brazilian Congress.
- On March 23, 2023, an unprecedented decision by the Courts in Sergipe (a Brazilian state) authorized the nonprofit association Salvar (Brazilian Association for the Support of Medicinal Cannabis Cultivation and Research) to “carry out the cultivation, manipulation, preparation, production, storage, transport, dispensation and research of cannabis sativa […] for the exclusive treatment of its members”, according to doctor’s prescription. It is the first authorization given by a Brazilian Court for the cultivation and commercialization of cannabis flowers, extracts and edibles in the national territory.
- On April 19, 2023, the Oswaldo Cruz Foundation (Fundação Oswaldo Cruz – Fiocruz), which is linked to the Ministry of Health, launched a technical note setting out the scientific evidence found so far on therapeutic treatments performed with cannabis and its derivatives. The objective of the document is to offer technical support to the institutions responsible for legislation, regulation, research, production, standardization, distribution and use of cannabis and derivatives for therapeutic purposes in Brazil, as well as for population in general.
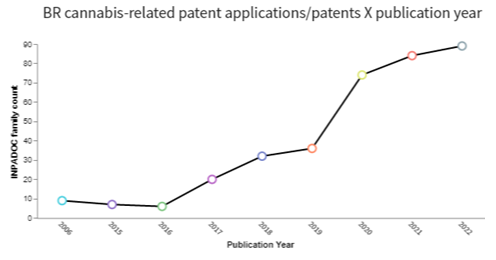
In the realm of intellectual property, it is notable that the number of published patent applications has increased considerably since 2019 (see the charts below), reflecting a greater investment in cannabis-related technologies in recent years. As can be seen from the charts below, the Brazilian scenario has followed the global scenario, with a notable increase in patent applications from 2019, but in a smaller proportion.
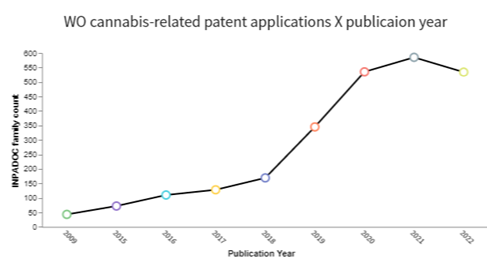
Source: Software Derwent™ Innovation, considering the keywords: cannabis OR cannabidiol
Due to the favorable climate for cannabis cultivation in Brazil, the flexibility in the cultivation of this plant could be considered a key factor to boost Brazil in the cannabis-based medicines industry, contributing to reduced production costs related to the raw materials (which are mostly imported) and, in consequence, encouraging production, as well as research and development by national pharmaceutical companies in this promising field.
Finally, the flexibility of the commercialization of these medicines (like in the United States) could leverage the investment of foreign companies in the Brazilian market, which, rationally, should increase the demand for patent and trademark protection for these technologies in the country.
Image Source: Deposit Photos
Image ID: 313374524
Author: [email protected]
Kene Gallois
is Head of the Chemical & Life Sciences Group at Daniel Law. Kene has more than 10 years experience in the field of Biological Sciences. She graduated from the Fluminense […see more]
Samantha Salim
Samantha Salim has been working with Intellectual Property since 2010 and joined Daniel Law as a patent specialist in 2015. Her expertise covers all aspects related to the prosecution of […see more]