Mylan Pharmaceuticals Inc. v. Merck Sharp & Dohme Corp. (Fed. Cir. 2022) | McDonnell Boehnen Hulbert & Berghoff LLP
The Federal Circuit affirmed the Patent Trial and Appeal Board’s (PTAB) Final Written Decision (FWD) in an inter partes review (IPR) that Mylan Pharmaceuticals failed to show the claims of U.S. Patent No. 7,326,708 were either anticipated or rendered obvious by the asserted prior art, in Mylan Pharmaceuticals Inc. v. Merck Sharp & Dohme Corp.
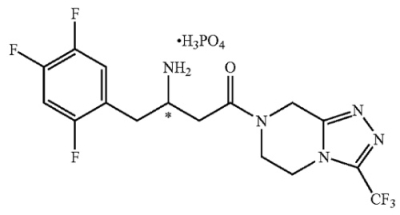
The claimed subject matter at issue was sitagliptin dihydrogenphosphate (“sitagliptin DHP”), a dihydrogenphosphate salt of 4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro [1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine:
This compound is useful for treating non-insulin dependent (Type 2) diabetes based on its activity as a dipeptidyl peptidase-IV inhibitor. Claims 1-4, 17, 19, and 21-23 of U.S. Patent No. 7,326,708 were challenged in the IPR.
Mylan argued that International Patent Publication No. WO 2003/004498 (equivalent to another Merck patent, U.S. Patent No. 6,699,871) anticipated the claims at issue in the IPR. Specifically, the ‘498 application disclosed dipeptidyl peptidase-IV inhibitors for treatment of diseases such as Type 2 diabetes. The application disclosed 33 species of these inhibitors, including sitagliptin. Also disclosed were the preparation of pharmaceutically acceptable salts (as a class) and hydrates thereof, including but not specifically directed to phosphoric acid. The Board rejected Mylan’s anticipation argument because the ‘498 application did not disclose all of the limitations in the challenged claims (specifically a 1:1 sitagliptin DHP salt), based inter alia on “evidence, both experimental and from the technical literature, undeniably showed that 1:1 sitagliptin DHP does not form every time sitagliptin and DHP were reacted” (which was the basis for the Board rejecting Mylan’s inherent anticipation argument).
The Board also rejected Mylan’s obviousness assertions, based on the combination of the ‘498 application with two scientific publications: Brittain, which disclosed the “pharmaceutical importance and prevalence of crystalline hydrates of pharmaceutical compounds”; and Bastin, which disclosed “salt selection and optimization procedures during the development of pharmaceutical compounds.” For claims 1, 2, 17, 19, and 21-23 the Board held that Merck was able to antedate the ‘498 application under pre-AIA 35 U.S.C. § 102(a), and while the related ‘871 patent was available as prior art under pre-AIA § 102(e), application of the reference was prohibited under pre-AIA § 103(c) because the ‘871 and ‘708 patents were commonly owned or subject to an obligation for assignment.
With regard to claim 3 (directed to the (S)-configuration of sitagliptin) and claim 4 (directed to the (R)-configuration), the Board found that none of the cited references disclosed (S)-sitagliptin and thus claim 3 was not obvious. Regarding (R)-sitagliptin recited in claim 4 the Board found that “Mylan provided no rationale to explain why a person of ordinary skill would have been motivated to make the claimed crystalline monohydrate form of 1:1 sitagliptin DHP of claim 4 and failed to show that a skilled artisan would have had a reasonable expectation of success in making the crystalline monohydrate form of the 1:1 sitagliptin DHP salt.”
The Federal Circuit affirmed in an opinion by Judge Lourie, joined by Judges Reyna and Stoll. Regarding Mylan’s anticipation argument, the Court held the Board’s decision was supported by substantial evidence, based in part on the testimony of Mylan’s own expert witness that the ‘498 application did not expressly disclose sitagliptin DHP nor the production of dihydrogenphosphate salts. The panel also found substantial evidence supported the Board’s determination that the ‘498 application did not inherently anticipate the sitagliptin dihydrogenphosphate claimed in the ‘708 patent. This decision distinguished the circumstances here from those in In re Petering (relied upon by Mylan), on the basis that Petering was based on their being only a limited number of members of the class of compounds. 301 F.2d 676, 681 (C.C.P.A. 1962). In this case, the evidence of record showed that the 33 disclosed dipeptidyl peptidase inhibitors could result in 957 combinations with various pharmaceutically acceptable salts, under circumstances where another Mylan expert admitted that “salt formation is an unpredictable art that requires a ‘trial and error process.'” While not ruling on what would or could constitute a limited class that would satisfy the Petering standard the Court held that the proper standard was not met under these circumstances.
In considering the Board’s decision on obviousness grounds, the Court first considered and rejected Mylan’s argument concerning whether Merck’s experimental evidence and the status of the ‘871 and ‘708 patents under pre-AIA §§ 102(a) and 102(e) precluded assertions of the ‘498 application as prior art. Once again, the Court held that the Board’s decision was supported by substantial evidence, including that the ‘498 application did not disclose 1:1 sitagliptin DHP and thus could not and did not disclose a monohydrate of the salt.
Finally, with regard to claims 3 ((S)-sitagliptin) and 4 ((R)-sitagliptin), the panel also found the Board’s decision supported by substantial evidence. The FWD held that Mylan failed to establish a motivation to combine the ‘498 application and the Bastin reference and that the skilled artisan would not have had any reasonable expectation of success had the reference been combined. Considering claim 3 (again relying on Mylan’s expert’s testimony that the (S)-sitagliptin enantiomer was not disclosed in the ‘498 application, the panel held the Board was correct in concluding that Mylan had “advanced no expected or theoretical benefit to making the (S)-enantiomer of 1:1 sitagliptin DHP, and that the general disclosure on diastereomers in [the ‘498 application] encompasses millions of potential compounds and salts with no motivation to make the (S)-enantiomer with a reasonable expectation of success, particularly in an unpredictable activity like salt formation.” Similarly, the Court held the PTAB’s decision regarding claim 4 was also supported by substantial evidence that there would have been no motivation to combine the cited references nor any reasonable expectation of success in doing so (yet again relying on Mylan’s expert’s testimony in this regard and also Merck’s expert regarding the art teaching away from hydrates due to their unpredictability). (In an aside, the opinion also affirms the Board’s consideration of secondary indicia of non-obviousness).
While there is nothing particularly earth-shattering or precedent-setting in the Court’s precedential opinion, it does illustrate once again the difficulty parties have in overcoming the presumption of substantial evidence in IPRs (and the advantages to be accrued by attempting to avoid IPR institution in the first place).
Mylan Pharmaceuticals Inc. v. Merck Sharp & Dohme Corp. (Fed. Cir. 2022)
Panel: Circuit Judges Lourie, Reyna, and Stoll
Opinion by Circuit Judge Lourie