FDA Approves Fresenius Kabi Biosimilar for Neulasta® | McDonnell Boehnen Hulbert & Berghoff LLP
It was not so long ago that many, including members of Congress, were bemoaning the slow approval and introduction into the marketplace of biosimilar alternatives to (generally expensive) biologic drugs (see “Trump Administration Considering Reduction in Biologics Exclusivity Period; A Solution in Search of a Problem”; “FDA Issues Plan for Further Facilitating Biosimilar Development”; “Senators Ask FTC to Investigate Biosimilar Litigation Settlement Agreement”; “Biosimilars Action Plan: Balancing Innovation and Competition”; “Congress Jumps on Bandwagon to Reduce Biologic Drug Exclusivity Term”). These sentiments were largely from outcome-oriented groups with little understanding or regard for process or an appreciation of what has been required of the FDA and biosimilar applicants to bring safe and effective biosimilar drugs to market (and to be sure the same chorus would be vocally critical should a biosimilar fail to be safe, effective, and cheaper than the reference biologic drug product).
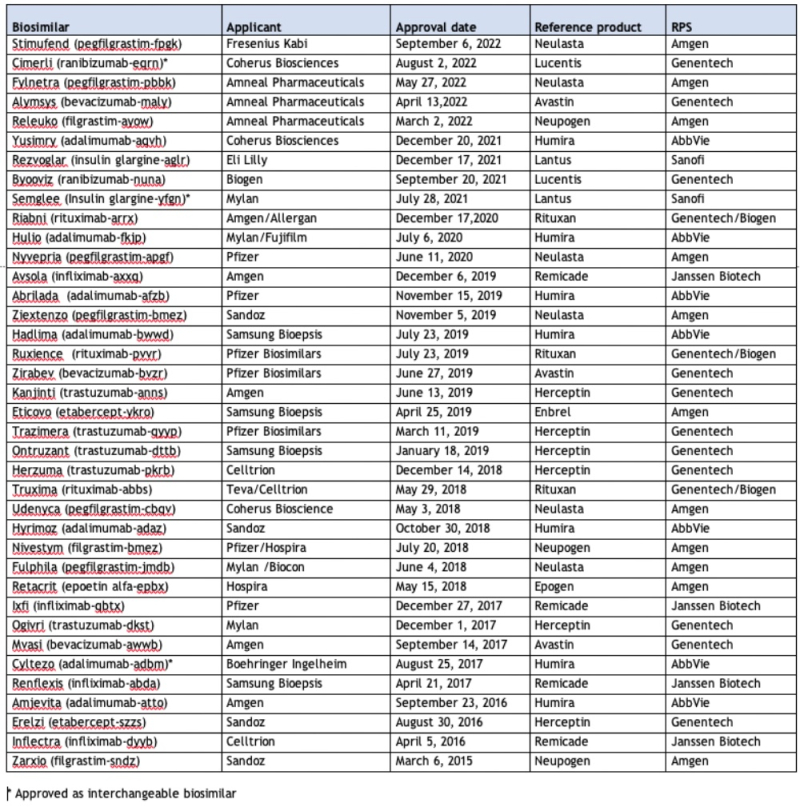
Things are looking up, however. On September 6th, Fresenius Kabi announced FDA approval for its Stimufend® (pegfilgrastim-fpgk) product, as an interchangeable biosimilar to Amgen’s Neulasta® (pegfilgrastim). This biologic drug is a recombinant human granulocyte colony-stimulating factor (or G-CSF) approved for decreasing the incidence of infection, as manifested by febrile neutropenia, in patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of febrile neutropenia. Fresenius Kabi plans on marketing the product in a prefilled syringe by early 2023, and further plans to develop an “on-body injector” product to compete with Amgen’s Neulasta Onpro (which has captured 45% of the $3.1 billion Neulasta® market as of the fourth quarter 2019). Previously, Fresenius Kabi received marketing authorization in March 2022 for its Stimufend® product in Europe.
This approval brings to 38 the number of approved biosimilar products.
[View source.]





