Does Hyatt v. Hirshfeld Mean That More than One-Third of Patents on the Top Pharmaceuticals are Presumed Invalid?
“If a patent system is structured such that the judicial branch can retroactively presume that one-third of patents protecting the top pharmaceuticals are invalid, inventors and companies may struggle to believe that the quid pro quo provided by the system is not a scam.”
Case law has defined prosecution laches as an affirmative defense against an infringement assertion. Specifically, the case law indicates a patent that is being asserted is unenforceable when the patentee caused an unreasonable and unexplained delay in prosecution of the patent. Symbol Tech v Lemelson Medical, No. 04-1451 (Fed. Cir. 2005). There is relatively little case law on the specifics of laches. However, in 2021, the Federal Circuit said: “we now hold that, in the context of a § 145 action, the PTO must generally prove intervening rights to establish prejudice, but an unreasonable and unexplained prosecution delay of six years or more raises a presumption of prejudice”. Gil Hyatt v. Hirshfeld (Fed. Cir. 2021). What does this – or might this – mean beyond the Hyatt case?
The pharmaceutical area may provide an insightful perspective on the potential reach of this dicta, given that pharmaceutical patents are frequently relied upon to at least partly justify billions of dollars of investment for research and development. Therefore, this study investigates patents that were listed in the Orange Book or Purple Book as protecting the top 20 pharmaceuticals, in terms of revenue.
Drilling Down on the Data
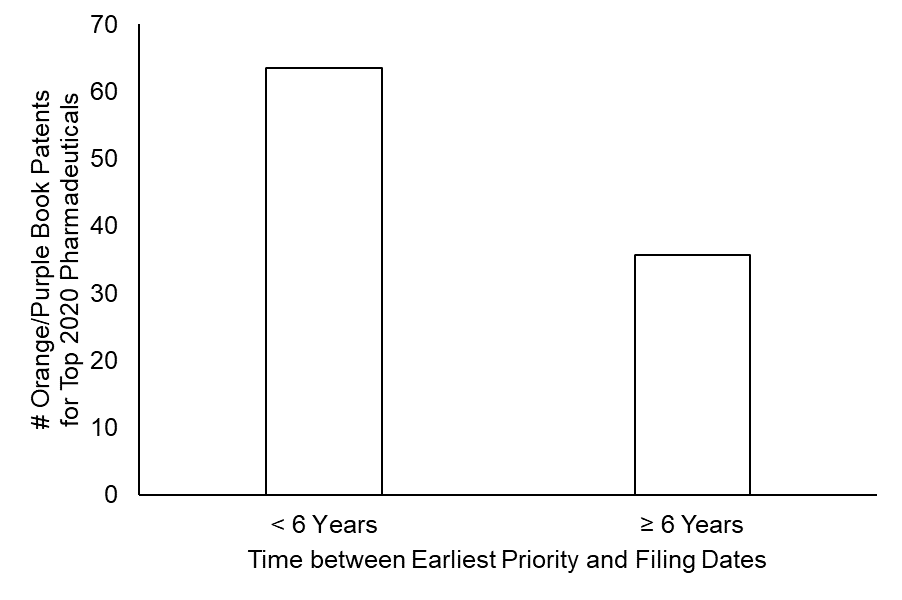
More specifically, the 20 pharmaceuticals with the highest 2020 revenues were studied. For each of these drugs, IPDataLab identified each patent listed in the Orange Book (which includes information about approved small-molecule therapeutics) or the Purple Book (which includes information about approved biologic therapeutics) that had been identified by the pharmaceutical company as protecting the drug. For each patent application, the earliest priority date and the application’s filing data were also identified to determine the time difference between the priority date and the application’s filing date.
As shown in FIG. 1, over one-third of the patents (87 out of 244) that were identified as protecting the top-revenue pharmaceuticals had a delay of over six years between an earliest priority date and the application filing date.
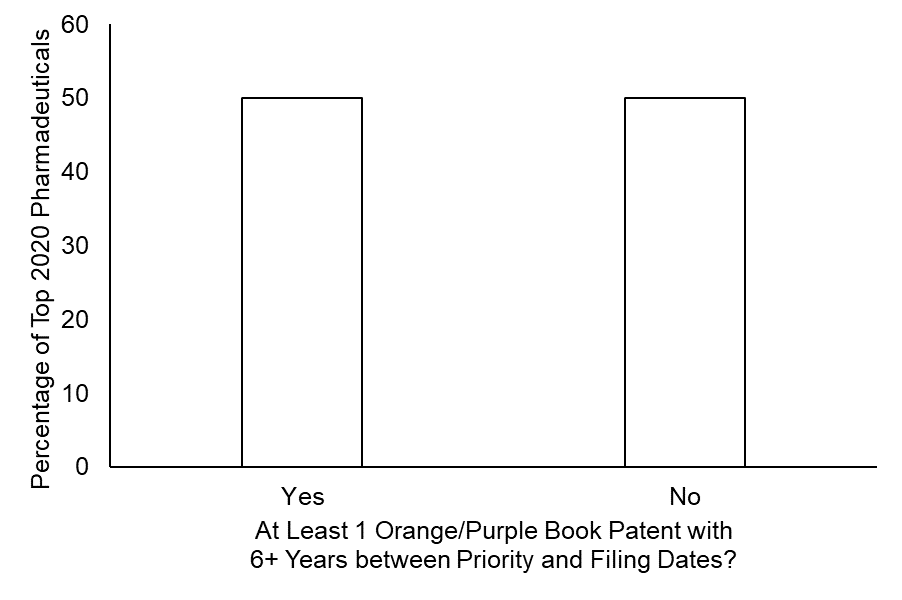
Notably, the size and temporal profile of patent portfolios did vary across these pharmaceuticals. However, of the pharmaceuticals with the highest 2020 revenue, 50% of these top pharmaceuticals had at least one patent listed in the Orange Book or Purple that – according to the dicta in the Hyatt case would be presumed to be invalid. (See FIG. 2.)
Anti-Innovation Implications
Recall that the Federal Circuit’s analysis of laches in Hyatt is tied to a “presumption of prejudice”. This analysis concentrates on whether there would be prejudice against the public, but particularly when an invalidity avenue is being defined by judges and not the legislative branch, it is important to also consider the potential impacts to the patent owner and overall fairness and societal efficiency.
From the patent owner’s perspective, U.S. Patent and Trademark Office (USPTO) fees (and likely attorney fees) were paid with the expectation that – if a patent application is allowed and complies with the statutory patent requirements –it will lead to a valid and enforceable patent. If a patent system is instead structured such that the judicial branch can retroactively presume that one-third of patents protecting the top pharmaceuticals are invalid, inventors and companies may struggle to believe that the quid pro quo provided by the system is not a scam and/or that an investment in researching and developing a product is a sound business decision.
Further, consider the approval timeline for a pharmaceutical. An entity may discover that a particular small- or large-molecule drug shows promise for effectively treating a medical condition, and they may quickly file a patent application. However, then years of clinical trials are performed to ensure that the drug truly does effectively treat the condition, that any side effects are not so severe and/or common to outweigh the drug’s benefit, to identify appropriate patient groups, to identify specific dosages, etc. Subsequently, or in the meantime, the entity may submit and pursue an application to the Food and Drug Administration to request approval of the drug. In the vast majority of instances, a promising pharmaceutical candidate is dropped in response to sub-optimal experimental or trial data. The Federal Circuit’s proposed presumption of prejudice if there is a more than six-year delay between a priority date and a filing date may put the entity in a position of having to choose between initially investing even more money in each pharmaceutical candidate for patent protection (even though the vast majority of drug candidates fail) or potentially being in a position where the patent protection for a highly valuable pharmaceutical is weak. Thus, the laches presumption sets up a situation that promotes even larger investments in research efforts that are statistically very expensive and very likely to fail.
Thus, in sum, the Federal Circuit’s presumption of laches applying with an application that is filed more than six years after a priority date would have a large impact on existing pharmaceutical patents. Judicially creating new invalidity presumptions that apply retroactively is a very risky move, as courtrooms are not set up to ensure that diverse interests are well represented or that the potentially chilling effects are thoroughly considered.
Image Source: Deposit Photos
Image ID: 204633772
Author: Alexis84
Kate Gaudry
Kate Gaudry is a partner at the Washington, D.C., office of Kilpatrick Townsend & Stockton LLP, where she focuses her practice on data-driven and strategic patent prosecution. She has authored over […see more]