Chromadex, Inc. v. Elysium Health, Inc. (Fed. Cir. 2023) | McDonnell Boehnen Hulbert & Berghoff LLP
Judge Giles Sutherland Rich, famous for many things (including being the principal author of the 1952 Patent Act and in particular Section 103, which cabined at least for a while the Supreme Court’s penchant for invalidating patents to such an extent that Justice Jackson remarked that the only valid patent was one the Court had not had an opportunity to invalidate, Jungersen v. Ostby & Barton Co., 335 U.S. 560, 572 (1949)) is perhaps most well-known for his aphorism that “the name of the game is the claim.” An illustration of the wisdom of this phrase is the recent decision by the Federal Circuit affirming the District Court’s invalidation of asserted claims on subject matter eligibility grounds in Chromadex, Inc. v. Elysium Health, Inc.
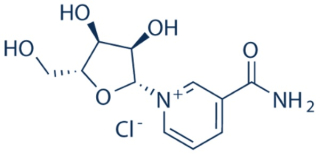
The case arose over U.S. Patent No. 8,197,807, directed to dietary supplements comprising nicotinamide ribonucleotide (which is converted in the body to nicotinamide adenine dinucleotide, or NAD+), a co-enzyme commonly known as vitamin B3:
Representative claim 1 at issue was set forth in the opinion:
1. A composition comprising isolated nicotinamide riboside in combination with one or more of tryptophan, nicotinic acid, or nicotinamide, wherein said combination is in admixture with a carrier comprising a sugar, starch, cellulose, powdered tragacanth, malt, gelatin, talc, cocoa butter, suppository wax, oil, glycol, polyol, ester, agar, buffering agent, alginic acid, isotonic saline, Ringer’s solution, ethyl alcohol, polyester, polycarbonate, or polyanhydride, wherein said composition is formulated for oral administration and increased NAD+ biosynthesis upon oral administration.
After construing the term “isolated nicotinamide ribonucleotide” (or “[NR]”) to mean “[NR] that is separated or substantially free from at least some other components associated with the source of [NR],” the District Court granted summary judgment to defendant Elysium for failure of the claims to recite patent eligible subject matter under 35 U.S.C. § 101, on the grounds that isolated nicotinamide ribonucleotide is a product of nature declared ineligible under the rubrics set forth by the Supreme Court in Ass’n for Molecular Pathology v. Myriad Genetics, Inc., 569 U.S. 576, 589 (2013).
The Federal Circuit affirmed in an opinion by Judge Prost joined by Judges Chen and Stoll. The opinion sets forth a comparison between the elements of the claim and milk, a natural product the panel considered manifestly patent ineligible as a product of nature:
The opinion characterizes the claims as being “very broad” and to read on milk with only one difference, i.e., that the nicotinamide ribonucleotide is not isolated.
The Federal Circuit relied on the Supreme Court’s Myriad decision and contrasted it with the Court’s decision in Diamond v. Chakrabarty, 447 U.S. 303, 309 (1980), in reaching its decision. In Chakrabarty, the claimed microorganism was not naturally occurring, having been genetically manipulated to encode enzymes capable of digesting “multiple components of crude oil,” inter alia, in oil spills, citing as the standard that the claimed bacterium was “a nonnaturally occurring manufacture or composition of matter—a product of human ingenuity having a distinctive name, character and use.” Here, as in Myriad, the claimed nicotinamide ribonucleotide while having been “isolated” was not different and distinct enough “structurally or functionally from its natural counterpart in milk” to be patent eligible. The Federal Circuit recognized in this opinion one important distinction expressly introduced into subject matter eligibility considerations for natural products by the Supreme Court in its Myriad opinion, that “mere” isolation is not enough to confer eligibility. This rubric was at odds with how Judge Rich understood natural product eligibility to be determined; after all, in his most famous eligibility case (the companion to Chakrabarty, a case captioned In re Bergy) there was no question raised that “merely” isolated lincomycin antibiotic was eligible.
The opinion also references another post-Myriad natural product opinion, Natural Alternatives Int’l, Inc. v. Creative Compounds, LLC, 918 F.3d 1338, 1342 (Fed. Cir. 2019), where the Federal Circuit held the claims patent eligible. In Natural Alternatives, the Court recognized that claims to a natural product (beta-alanine) were patent-eligible for reciting “specific treatment formulations that incorporate[d] natural products” and that those formulations “ha[d] different characteristics and c[ould] be used in a manner that beta-alanine as it appears in nature cannot.” Moreover, in that case “natural products ha[d] been isolated and then incorporated into a dosage form”—”between about 0.4 grams to 16 grams”—”with particular characteristics”—namely, to “effectively increase[] athletic performance.” The Court found those “markedly different characteristics distinguished the claimed supplements from natural beta-alanine and preserved the claims’ validity.”
Those distinctions made all the difference for the Federal Circuit, wherein the opinion noted that the distinctions argued by Chromadex did not require that the claimed composition contained any minimum quantity of nicotinamide ribonucleotide or that the claimed composition increased NAD+ biosynthesis (something that milk itself also did). The primary deficiency recognized by the panel is that “the claims simply do not reflect the distinctions Appellants rely on,” including that “they do not require any specific quantity of isolated NR” and that the term nicotinamide ribonucleotide as construed by the District Court did not require purification from lactalbumin whey protein argued as a distinction by Chromadex.
Having arrived at its determination, the panel went on to apply the formalism of the two-part test enunciated by the Supreme Court in Mayo Collaborative Servs. Prometheus Labs., Inc., 566 U.S. 66, 77–80 (2012), and Alice Corp. Pty. Ltd. v. CLS Bank Int’l, 573 U.S. 208, 217 (2014), in support of its decision. Not surprisingly, the Federal Circuit came to the same conclusion, finding reliance on the “natural law” that nicotinamide ribonucleotide compositions cause an increase in NAD+ in vivo.
There may be some comfort for the patentee in that the application resulting in this patent has an earliest priority date of April 20, 2005, many years prior to the Supreme Court’s Myriad decision, or that there is another patent in the family, U.S. Patent No. 8,383,086, that claims oral pharmaceutical formulations of nicotinamide ribonucleotide. But an analysis that patent lawyers have been doing long before Myriad’s enhanced considerations of subject matter eligibility were those concerning novelty and non-obviousness. The table contained in the opinion suggests that the claims had “bare-bones” novelty at best and the lone distinction, incorporation of “isolated” nicotinamide ribonucleotide, appears a thin reed to support an assertion of non-obviousness. While this is just another illustrative example of how the Court could have reached the policy goals in Mayo and Alice (and perhaps Myriad, come to that) using other portions of the patent statute, it also shows that Justice Breyer’s apprehension about the “clever draftsman” were both warranted and perhaps the best (only?) bulwark innovators have against summary invalidation of their patents under Section 101.
Chromadex, Inc. v. Elysium Health, Inc. (Fed. Cir. 2023)
Panel: Circuit Judges Prost, Chen, and Stoll
Opinion by Circuit Judge Prost




![Intellectual Property Rights and the Rule of Law [2020 National Lawyers Convention] Intellectual Property Rights and the Rule of Law [2020 National Lawyers Convention]](https://americanlegaljournal.com/wp-content/uploads/2022/06/l-property-rights-and-the-rule-of-law-2020-national-lawyers-convention-CNtP6xxAOaE-390x205.jpg)
