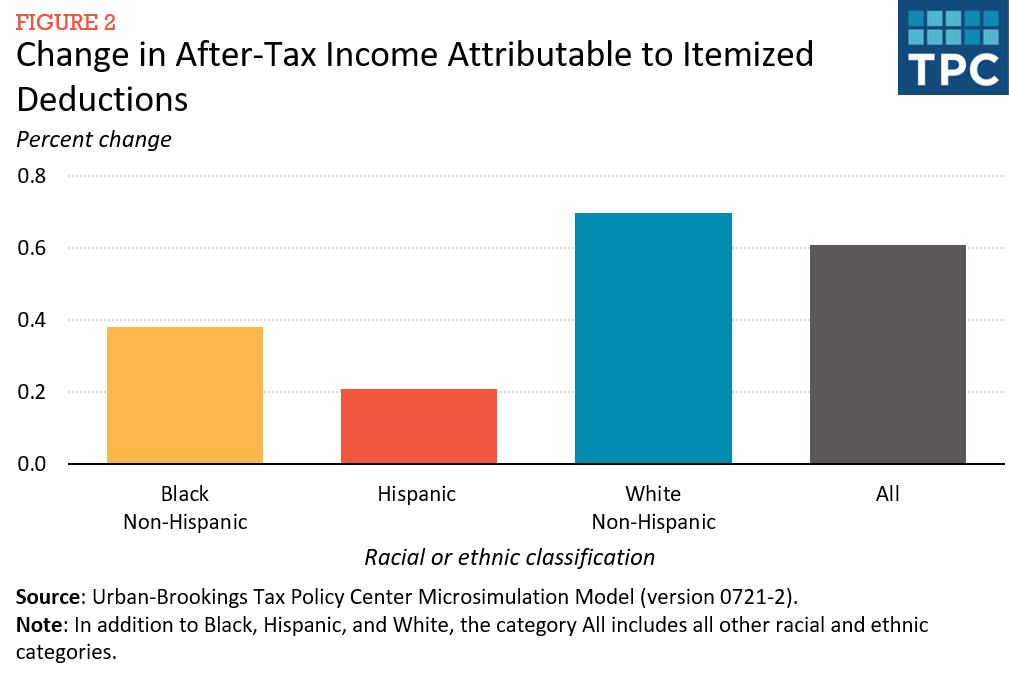
CAFC Says ChromaDex Patent Claiming Isolated Form of Vitamin B3 Fails Under 101
“As in Myriad, under the circumstances presented here, the act of isolating the NR compared to how NR naturally exists in milk is not sufficient, on its own, to confer patent eligibility.” – CAFC
The U.S. Court of Appeals for the Federal Circuit (CAFC) today issued a precedential decision affirming a Delaware court’s grant of summary judgment for Elysium Health that the asserted claims of ChromaDex, Inc.’s patent on an isolated form of vitamin B3 are directed to unpatentable subject matter under Section 101. Judge Prost authored the opinion.
U.S. Patent No. 8,197,807 is titled “Nicotinamide riboside kinase compositions and methods for using the same.” Nicotinamide riboside (NR) is a form of vitamin B3 that is naturally present in cow’s milk. The invention covers a composition containing isolated NR that results in increased biosynthesis of the coenzyme nicotinamide adenine dinucleotide (NAD+) upon oral administration.
ChromaDex sells dietary supplements embodying the patent, which it licenses from Dartmouth College, and sued Elysium for patent infringement in 2018. Elysium moved for summary judgment that the claims were invalid under Section 101 and the district court agreed, finding that the claims were directed to a natural phenomenon, specifically, “compositions comprising isolated [NR], a naturally occurring vitamin present in cow milk.”
Nothing ‘Markedly Different’ to See Here
In its discussion, the CAFC agreed with the U.S. District Court for the District of Delaware that the claims are directed to a product of nature under Ass’n for Molecular Pathology v. Myriad Genetics, Inc. and Diamond v. Chakrabarty. “As in Myriad, under the circumstances presented here, the act of isolating the NR compared to how NR naturally exists in milk is not sufficient, on its own, to confer patent eligibility,” wrote the court. The proper inquiry was defined in Chakrabarty, the opinion continued, where the Supreme Court said that, “to be patentable, the claimed composition must ‘ha[ve] markedly different characteristics and have the potential for significant utility.’” But naturally occurring NR in milk increases NAD+ biosynthesis when taken orally, just like the claimed invention.
Distinguishing the case from Natural Alternatives Int’l, Inc. v. Creative Compounds, LLC, the CAFC explained that the patent here is not markedly different from milk. While the appellants claimed the compositions are preferable to milk because isolating the NR allows for greater NAD+ biosynthesis than found in milk alone and that the isolated NR alone can increase NAD+ biosynthesis, the CAFC said that 1) the claims lack a minimum required quantity of isolated NR and 2) the claims attribute the increase in NAD+ only to the composition, rather than the isolated NR alone. The court further explained:
“To be sure, the claims cover several different composition embodiments, some of which are structurally different from milk. However, as noted above, the claims also encompass—as both parties agree—at least one embodiment that covers milk, except that the NR element is ‘isolated.’ Because the claims are broad enough to encompass a product of nature, it is invalid under § 101.”
ChromaDex argued the claims do possess markedly different characteristics from milk, including 1) that “NR is found in milk in only trace amounts”; and (2) “what little NR is found in milk is not bioavailable” as it is bound to lactalbumin whey protein. The Federal Circuit explained that, despite the trace amounts of NR, milk does increase NAD+ biosynthesis, due to the presence of tryptophan. Since the only therapeutic effect required by the patent claims is increased NAD+ biosynthesis, there is no marked difference. Secondly, the court said the district court’s unchallenged construction of “isolated NR” does not require that the NR be separated from the lactalbumin whey protein but only from “some of the other components associated with the source of [NR].”
No Help from Alice-Mayo
While the court noted that the discussion could end there, it next addressed the relevance of the two-part Alice-Mayo analysis. “[I]f resort to Alice/Mayo is necessary, then at step one we conclude the asserted claims are directed to a product of nature for the reasons stated above, and at step two the claims lack an inventive step because they are directed to nothing more than compositions that increase NAD+ bio-synthesis, which is the very natural principle that renders the claims patent-ineligible,” said the court.
The court dismissed ChromaDex’s assertions that recognizing the benefits of NR on health and the wisdom of isolating the NR to increase concentrations compared to those that occur naturally in milk represented inventive steps. The opinion concluded:
“[R]ecognizing the utility of NR is nothing more than recognizing a natural phenomenon, which is not inventive…. And the act of isolating the NR by itself, no matter how difficult or brilliant it may have been (although the specification makes clear that it was conventional), similarly does not turn an otherwise patent-ineligible product of nature into a patentable invention. So the claims would likewise fail at step two.” [citations omitted]
Elysium and ChromaDex have been engaged in litigation on several fronts since 2016.
Eileen McDermott
Eileen McDermott is the Editor-in-Chief of IPWatchdog.com. Eileen is a veteran IP and legal journalist, and no stranger to the intellectual property world, having held editorial and managerial positions at […see more]