Analysis of Patent Applications for Novel Coronavirus Detection Kits | Linda Liu & Partners
[Authors: Wang Jing and Liuxin Jin]
The rapid spread of the novel coronavirus (officially known as “SARS-CoV-2” and hereinafter referred to as “NC” for short) led to the COVID-19 pandemic. All countries around the world are fighting against the disease. In this fight, detecting the virus accurately and rapidly is one of the important means to preventing its spread. Thanks to the research endeavors made by some of China’s enterprises engaged in in vitro diagnosis and to the approval green paths set up by the drug regulatory authority, there are at present seventy-two NC detection kits that have been registered at the National Medical Products Administration and been available on the market (the number being calculated by the codes of the registration certificates).
The following is an introduction of patent applications filed with the China National Intellectual Property Administration (CNIPA) that are related to NC detection kits.
We have searched in databases using keywords and retrieved, as of June 10, 2022, a total of 1,437 China patent applications related to NC detection kits, which include those merely mentioning the word “kit” and the majority of which are directed to proteins and sequences thereof and to improvements on preparation methods. Now, let’s have a look at those patent applications from different points of views.
Overview of applications
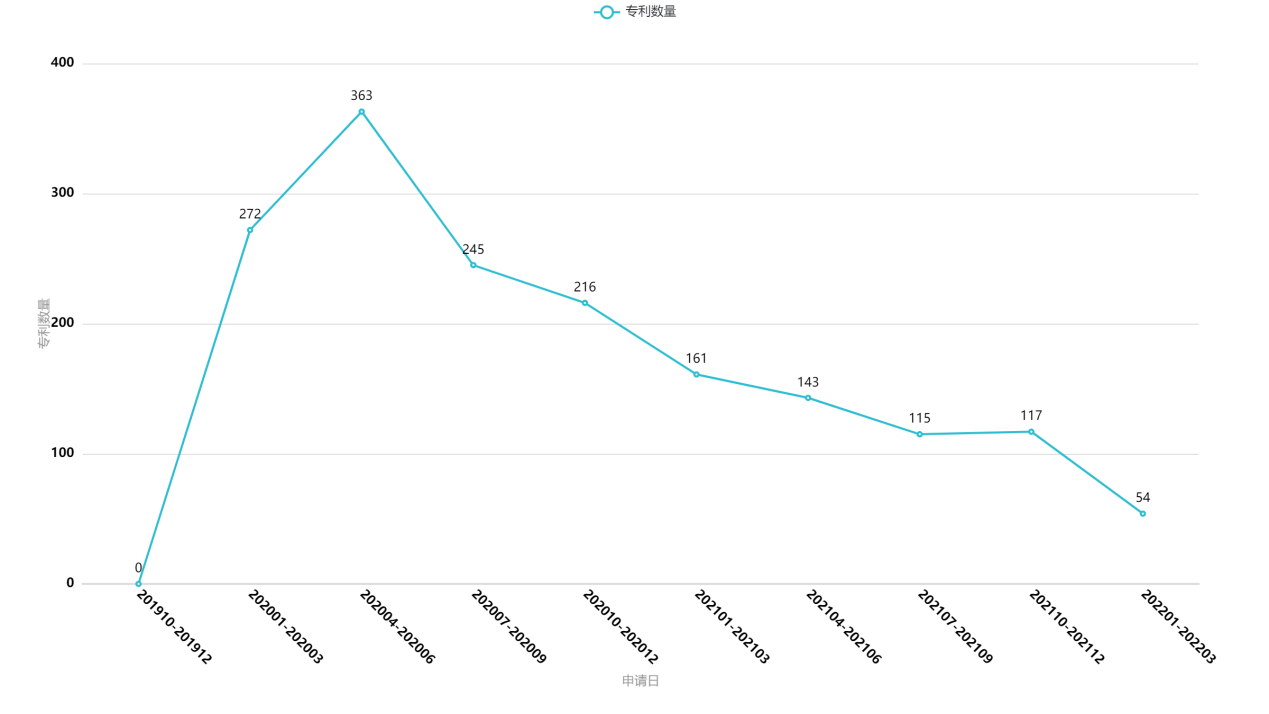
Fig. 1 Change in Number of Applications over Time
Fig. 1 illustrates the relationship between the filing dates (on a quarterly basis) and the number of the applications. As shown in Fig. 1, the number of the applications peaked about 4 months after the outbreak of the pandemic (namely, in the second quarter of 2020). This is a showcase of how quickly Chinese researchers and enterprises responded to the pandemic and how efficiently they carried out the researches.
Of the applications, those for invention patent are the majority; 143 of them are for utility model patent, and 11 of them are for design patent. The applications for utility model patent are focused on improvements on physical structures such as kits, testing cards, and portable testing boxes. Those for design patent are primarily packaging boxes, in addition to a couple of ones concerning the configuration of kits and testing cards, e.g., the configuration of combined detection devices, saliva detection cards, and antibody kits for the NC or for influenza A and B viruses (see Fig. 2).
Fig. 2 Design Patent CN306504939S
NC detection covers a field of high technical values, so applicants have been seeking protection for it by principally filing applications for invention patent. For innovations in shape and configuration such as kits and testing cards, however, applicants might as well try design patents to make future exercise of rights convenient because verbal expressions of the claims in invention and utility model patents have their limitations.
Trend of examination
Of all the applications, nearly 450 have been patented, more than 950 are under examination, more than 20 have been withdrawn, and 19 have been finally rejected. The proportion of the number of the patented to the total number of the patented, the withdrawn and the rejected is greater than 90 %, which is evidence that NC detection is a highly innovative field.
Fig. 3 Time Taken by Examination of Applications before Patented
As shown in Fig. 3, more than half of the applications that got patented had been in examination for a period of 1 to 6 months. According to the CNIPA’s 2021 annual report, the time for examining applications for invention patent in the second half of 2021 averaged out at 18.5 months per application. Clearly, the time for examining applications related to NC detection kits was short, with the shortest time being only two months from the date when the application was filed to the date when a patent was granted on it. This was due to the fact that the CNIPA specially opened a green path for the speedy examination of patent applications related to COVID-19.
Leading applicants
Fig. 4 Leading China Applicants
Institute of Microbiology of the Chinese Academy of Sciences
The Institute of Microbiology of the Chinese Academy of Sciences (IMCAS), as a representative of research institutes, have filed 20 patent applications related to NC kits with the CNIPA, of which 6 have been patented, and 14 are in examination. It has filed 4 of the applications with the Hong Kong and Taiwan patent offices and with the World Intellectual Property Organization (WIPO).
The IMCAS has focused its research on fundamental aspects of COVID-19 such as antibodies, epitopes, neutralizing antibodies, vaccines, and medicaments, and it mentioned in its applications use of its research in detection and diagnostic kits. Those applications are mostly shared by cooperative applicants.
In December 2020, Gao Fu team and Jiangsu Meike jointly developed a kit for detecting neutralizing antibodies rapidly and accurately (by a colloidal gold approach) in order to effectively screen out the population to be vaccinated and efficiently monitor immune effects of the vaccinated population.
As the virus continues to spread and variants thereof emerge, there is a need for antibodies or fragments thereof as well as vaccines and kits derived from them that can offset a decrease in the neutralizing potency of the previous antibodies that was caused by virus mutations and that are still effective against the variants. In February 2021, Gao Fu team discovered “Human ACE2 Modified Protein and ACE2-hFc-like Antibody for SARS-CoV-2.” The team disclosed use of such a fusion protein in the preparation of a reagent and kit for detecting the presence of the variants in samples. Taking advantage of the priority right system, the IMCAS had refined its applications over one year. Many of its applications filed in 2021 claim the priority of its earlier applications. For example, the application titled “2019-nCoV N Protein Linear Epitope Peptide and Monoclonal Antibody, and Use of the Same in Detection Kit,” filed in February 2021, claims the priority of the early filing date of February 4, 2020. The application titled “Bispecific Antibody against SARS-CoV-2” (immune double antibody), filed in July 2021, claims the priority of the early filing date of July 31, 2020, which the applicant alleges can improve the selectivity and neutralizing activity of maternal monoclonal antibodies. In addition to the antibodies, the IMCAS has also expanded its research objects to medicaments that are effective in treating or alleviating COVID-19. It applied, together with the National Institute for Viral Disease Control and Prevention (NIVDC) under the Chinese Center for Disease Control and Prevention (CDC), for patents for use of the compound GC376/Boceprevir in treating or alleviating COVID-19 (priority date of which is April 24, 2020). The two applications describe synergism between the compound GC376/Boceprevir and Remdesivir.
In June 2021, other researchers at the IMCAS discovered “Methods for Detecting Nucleic Acids of Variants of SARS-CoV-2,” which is different from the research focus of Gao Fu team. Aimed at nucleic acid detection kits, that method broke through the then technical bottleneck of single-base identification for which the fluorescent PCR technology does not work.
At the beginning of 2022, the IMCAS continued its cooperation with Jiangsu Meike and revealed a joint detection kit for the bi-epitope recognition of “S-RBD protein” and “N protein.” The kit is capable of distinguishing between the highly-transmissible Omicron variant and the highly-pathogenic Delta variant by recognizing the NC’s different epitopes using different antibodies.
Daan Gene Co., Ltd.
As a representative of enterprises, Daan Gene Co., Ltd. (hereinafter referred to as “Daan Gene”) has also achieved significant results in researches into the NC. It has filed 18 kit-related patent applications, of which 3 have been patented, and 15 are under examination. The corporation has also filed applications overseas, for example, with the EPO, the USPTO, and the WPO. One of its patents titled “SARS-CoV-2 (ORF1ab) Nucleic Acid Detection Kit” (filed in March 2020) won the 8th Guangdong Patent Gold Award. On April 15, 2022, the patent preliminary won the 23rd China Patent Gold Award after being reviewed by the CNIPA, indicating that its technological innovativeness was affirmed by a national level review. Daan Gene carried out comprehensive research into the entire nucleic acid detection process and developed a patent strategy for the whole detection process. Jiang Xiwen, the principal inventor, was conferred the title of “Guangzhou Outstanding Individual in Fighting against COVID-19” in December 2020.
After the NC’s genome was published in January 2020, it took only 2 months for Daan Gene to finish filing a series of patent applications for the detection candidates: the E gene, N gene and ORF1ab gene (the patent for which won the award). Subsequently, the applicant applied for patents for the multi-target detection and the relevant kits. From the dual-target (ORF1ab and N genes) patent, Daan Gene determined a dual-target approach, and had two 2019-nCoV nucleic acid detection kits (by a fluorescent PCR approach) registered. Of them, the kit with the registration code “国械注准20203400749” can complete the entire detection within one hour, making the detection convenient and fast.
Approved on 2021.1.21 Approved on 2021.4.2
国械注准20203400063 国械注准20203400749
Fig. 5 Two Nucleic Acid Detection Kits from Daan Gene
As a carrier of technologies, patents harbor the latest and most valuable part of technological innovation. An in-depth analysis of patent big data makes it possible to quickly find out scientific research teams and key enterprises in a target field. Comparing the various technical solutions of them leads you to make technological breakthroughs in the rapid and accurate detection of the virus.
The above preliminary analysis of the patents/patent applications of some of the representative applicants unfolds to us what they have researched and how they have devised a patent strategy for their researches, which enterprises, researchers and IP practitioners may consider for reference.









