Amgen Files Its Principal Brief in Amgen v. Sanofi | McDonnell Boehnen Hulbert & Berghoff LLP
Facing what is likely to be something of an uphill battle in seeking to have the Federal Circuit’s decision against it in Amgen v Sanofi overturned before a not always patent-friendly Supreme Court, Amgen in late December filed its opening brief addressing the Question Presented in the granted certiorari petition:
Whether enablement is governed by the statutory requirement that the specification teach those skilled in the art to “make and use” the invention, 35 U.S.C. § 112, or whether it must instead enable those skilled in the art “to reach the full scope of claimed embodiments” without undue experimentation ― i.e., to cumulatively identify and make all or nearly all embodiments of the invention without substantial “time and effort,” Pet. App. 14a [emphasis in Question].
The brief begins by disparaging the decision based on the Federal Circuit “once again” “imposing limitations on the Patent Act that are inconsistent with the Act’s text” as the Supreme Court asserted in Bilski v. Kappos, 561 U.S. 593, 612 (2010). Amgen argues that the focus of the Court’s consideration of the question before it should be on the statute and its text, which has not changed appreciably since the 1790 Patent Act enacted by the Founders. An important consideration regarding this question is the “patent bargain,” recognized by the Court in Universal Oil Prods. Co. v. Globe Oil & Refin. Co., 322 U.S. 471, 484 (1944), and Pfaff v. Wells Elecs., Inc., 525 U.S. 55, 63 (1998), and Amgen’s assertion that the Federal Circuit’s decision violates all three prongs — statutory text, precedent, and history — of enablement law in establishing its full scope” test (illustrating the incorrectness of the test in its view by asserting that patents protecting inventions like Watt’s steam engine and the Wright Brothers’ airplane would not pass muster under this test). “[T]he law has never required that, for those inventions to be patentable, skilled artisans must be able to cumulatively identify and make every variation without substantial time and effort” (emphasis in brief), Amgen argues and characterizes the standard as “folly” based on treatises from Robinson (1890) to the late Dmitriy Karshtedt’s paper entitled The Death of Genus Claims (Dmitry Karshtedt, Mark A. Lemley & Sean B. Seymore, The Death of the Genus Claim, 35 HARV. J. L. & TECH. 1, 23-35 (2021). The basis for the full scope test — the need to describe a patented invention through its full scope to satisfy enablement — is contrary to Supreme Court precedent, Amgen argues, citing (amongst many other cases as its argument is developed) Smith v. Snow, 294 U.S. 1, 11 (1935), for the principle that it is “not necessary to . . . describe in the specification[] all possible forms in which the claimed principle may be reduced to practice.” Amgen asserts that the proper alternative to the Federal Circuit’s test can be found in Consolidated Electric Light Co. v. McKeesport Light Co., 159 U.S. 465 (1895), “which applied the statutory standard to invalidate claims where there was proof that the patent’s instructions were not enabling for large classes of claimed subject matter.”
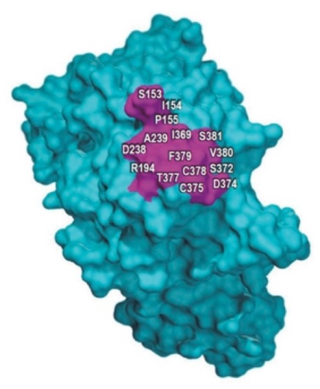
And such an analysis is not needed in this case, according to the brief, because there is no evidence that the disclosure is actually deficient in any way regarding Amgen’s “breakthrough” invention of monoclonal antibodies with real world benefits (reducing cholesterol). While setting forth an explication of the technology is unnecessary here, the brief emphasizes the limited area of the PCSK9 protein involved in the antibody interaction that provides the therapeutic benefit and constrains the antibodies that can interact with it, shown in this drawing as the purple-colored section of the molecule:
The brief emphasizes the extent of disclosure in the patent specifications instructing the skilled worker on how to make antibodies that recognize these limited portions of the protein, and the “roadmap” that teaches the skilled artisan how to make antibodies having the relevant therapeutic properties (many steps of which are “high throughput”), as well as the capacity to make “conservative” substitutions to provide alternative embodiments of antibodies having the requisite therapeutic function. The disclosure satisfies the Wands test, the brief asserts, and that should be enough.
In the face of this evidence from the specification (and the two juries’ fact-finding supporting their verdict that the claims were not shown to be non-enabled), Amgen argues that the Federal Circuit’s basis for invalidating for non-enablement was mere speculation, citing portions of the decision in support of these assertions stating that “there might be ‘millions of candidates’ that fall within the claims, each of which would have to be ‘generate[d] and then screen[ed]’ to determine whether it met the claims’ requirements”; “‘you could be immunizing mice for a hundred years,'” but “‘[t]here might be kind of an antibody that you didn’t come up with in that time period'”; and that those same conservative substitutions could produce (unpredictably) a non-functional antibody. Amgen emphasizes the contrariness of the Federal Circuit’s imposition of its invalidation on enablement grounds to the two juries’ decisions that the claims were enabled as well as the clear and convincing evidence standard of proof Amgen contends the juries’ decisions establish Respondent did not satisfy juxtaposed to what Amgen deems speculation by the Federal Circuit panel rendering the decision.
With regard to the statute, Amgen argues that its purpose is “to require the patentee to describe his invention so that others may construct and use it after the expiration of the patent,” citing Schriber-Schroth Co. v. Cleveland Tr. Co., 305 U.S. 47, 57 (1938), and that the full scope test has no basis in the statutory language. The statutory standard is straightforward, Amgen maintains, and evidence of this straightforwardness is that the Court has applied it without necessary interpretation (“directly”), citing Wood v. Underhill, 46 U.S. (5 How.) 1, 5 (1846) (permitting unspecified variations in types of clay used to make bricks and tiles); Mowry v. Whitney, 81 U.S. (14 Wall.) 620, 644 (1872) (method for manufacturing railway wheels that avoided “strain” due to different wheel parts cooling at different rates without requiring all types of wheels and all temperatures to be expressly exemplified); Loom Co. v. Higgins, 105 U.S. 580, 586 (1882); The Telephone Cases, 126 U.S. 1, 536 (1888); Minerals Separation, Ltd. v. Hyde, 242 U.S. 261, 271 (1916) (wherein a method for concentrating mineral ores using mixture and hearing with oil was permitted a scope that encompasses unexemplified ores and variations on the method to adapt it to different ores); and Universal Oil Prods. Co. v. Globe Oil & Refin. Co., 322 U.S. 471, 484 (1944). Amgen argues that the application of the standard in these cases is consistent with the plain meaning of the statute. On the contrary, the Federal Circuit’s “full scope” requirement deviates from the statutory text by requiring more than the statute does, by “focus[ing] on the “number of possible candidates within the scope of the claims”—the number of theoretical embodiments that might meet the claims’ requirements” (emphasis in brief). And this heightened requirement has not been applied to other genus claims Amgen argues, citing McRo inc. v. Bandai Namco Games Am. Inc., 959 F.3d 1091, 1100, as an example. What the Federal Circuit has done with its heightened “full scope” requirement is to have turned enablement into a “numbers game” with regard to the 26 exemplified disclosed antibodies and the theoretical “millions of candidat[e]” antibodies which would then need to be generate[d] and screen[ed]” to satisfy the full scope test. And this is an impossibility, Amgen states, as the Supreme Court has recognized in holding this was not the proper test, citing Mowry.
Amgen’s arguments harken back to the Framer’s adoption of English patent law (citing several English cases in which Amgen argues the full scope standard would have “flunked”), codified in the 1790 Patent Act that has remained essentially unchanged to the present 1952 statute. The consistency in the statute and its interpretation “tends to negate the existence of” the Federal Circuit’s new “full scope” standard Amgen argues, citing Printz v. United States, 521 U.S. 898, 918 (1997). 19th and early 20th Century U.S. case law is consistent with Amgen’s interpretation, the brief citing Carver v. Braintree Manufacturing Co., 5 F. Cas. 235 (C.C.D. Mass. 1843) (Justice Story) (cotton gins), as well as treatises (W. Phillips, The Law of Patents for Inventions 237 (1837); G. Curtis, A Treatise on the Law of Patents for Useful Inventions §124 (1849)) and regional Circuit Courts of Appeal decisions (Toledo Rex Spray Co. v. Cal. Spray Chem. Co., 268 F. 201, 204 (6th Cir. 1920); Donner v. Am. Sheet & Tin Plate Co., 165 F. 199, 206 (3d Cir. 1908); Philip A. Hunt Co. v. Mallinckrodt Chem. Works, 177 F.2d 583, 585 (2d Cir. 1949); Ill. Tool Works, Inc. v. Foster Grant Co., 547 F.2d 1300, 1309 (7th Cir. 1976)). Amgen also cites several pre-Federal Circuit cases that would have invalidated the patents at issue under the full scope test, including Franc-Strohmenger & Cowan, Inc. v. Arthur Siegman, Inc., 27 F.2d 785, 785-786 (2d Cir. 1928) (neckties made with “resilient” linings); and Ansul Co. v. Uniroyal, Inc., 448 F.2d 872, 877878 (2d Cir. 1971) (methods to grow plants encompassing “the entire plant kingdom”). Finally, the brief cites decisions from the Federal Circuit’s predecessor Court, the Court of Custom and Patent Appeals that (Amgen argues) support its “plain meaning” interpretation of the enablement requirements of Section 112, including In re Gay, 309 F.2d 769, 772 (C.C.P.A. 1962); In re Halleck, 422 F.2d 911, 912, 914 (C.C.P.A. 1970); and In re Angstadt, 537 F.2d 498 (C.C.P.A. 1976) (citing Minerals Separation).
Amgen further argues that the statute provides a “reasonableness” standard dependent on the patented subject matter that can balance the scope of the claims and whether the “specific instructions” are “sufficiently robust to permit skilled artisans to reasonably make and use individual embodiments as needed” rather than requiring a skilled artisan to be able to make “all embodiments, seriatim with minimal time and effort.” Amgen asserts that the Federal Circuit’s “full scope” standard “serves no valid patent-law policy and harms innovation[,] fundamentally alters the basic patent bargain[, and] discourages breakthrough innovations by cutting off patent protection for the most significant inventions simply because they have too many useful applications,” which “threatens devastating consequences.” The brief argues that the Federal Circuit’s full scope test is contrary to the constitutional mandate that the patent laws “promote progress” because “[o]nce an invention has been described sufficiently for skilled artisans to make and use it, disclosing thousands more examples of variations that achieve the same result contributes little to the store of human knowledge.” And worse, the brief argues, application of the test “destroys incentives for breakthrough inventions” like the case at bar, positing a correlation between “the more pioneering the innovation, the more likely it is to have a broad range of applications.” Perversely in Amgen’s view, the Federal Circuit test creates a situation where “[i]t makes no sense to deny groundbreaking innovations patent protection because they somehow have too many useful implementations” (emphasis in brief).
The brief also juxtaposes the practical application of the enablement test under well-established precedent under the statute with the “theoretical inquiry into hypothetical applications” engendered by the Federal Circuit’s full scope test. Defendants are not without means to challenge enablement under the conventional standard, Amgen asserts, as exemplified by instances where the skilled worker cannot practice a claimed invention at all, citing Beidler v. United States, 253 U.S. 447, 453 (1920), or that practicing the invention requires the public to achieve invention “in their own right” to obtain an operative embodiment, citing Consol. Elec. Light Co. v. McKeesport Light Co., 159 U.S. 465, 472-474 (1895), or reciting alternative embodiments in the claims where there is no teaching regarding one of them, citing Auto. Techs. Int’l, Inc. v. BMW of N. Am., Inc., 501 F.3d 1274, 1285 (Fed. Cir. 2007). There are also the “needle in a haystack” forms of non-enablement according to Amgen, such as in Idenix Pharms. LLC v. Gilead Scis. Inc., 941 F.3d 1149, 1162 (Fed. Cir. 2019), cert. denied, 141 S. Ct. 1234 (2021); and Wyeth & Cordis Corp. v. Abbott Labs., 720 F.3d 1380, 1384-1385 (Fed. Cir. 2013), or circumstances where the number of inoperative embodiments is so large that the effort required to produce an operative embodiment “becomes significant” as in Atlas Powder Co. v. E.I. du Pont De Nemours & Co., 750 F.2d 1569, 1576 (Fed. Cir. 1984); in all these instances the claims are not enabled under the conventional enablement test without need for recourse to the Federal Circuit’s full scope test. These cases also illustrate that there is no overbroad claiming “problem” in need of the full scope test to remedy, Amgen states, because “[i]f the claim truly exceeds what the patent enables, challengers will always be able to show, through evidence, that skilled artisans cannot reasonably ‘make and use’ large areas of the claimed invention by following the patent’s teaching” (emphasis in brief), as illustrated by the Court’s decision in Consolidated Electric, and several other cases, including Béné v. Jeantet, 129 U.S. 683 (1889); and Holland Furniture Co. v. Perkins Glue Co., 277 U.S. 245 (1928). Thus Amgen asserts the Federal Circuit’s full scope test is unnecessary in attempting to solve a problem that does not exist, and in doing so in this case “allow[ed] clearly enabled claims to be invalidated based on speculation rather than proof” as evidenced by Respondents’ failure “to identify even one actual antibody that could not be made following the patent’s disclosures” (emphasis in brief).
The brief takes the time (as it almost must) to set forth the deleterious consequences that are likely to arise if the full scope enablement test is affirmed. These include harm to genus claims not limited to chemical, biotechnological, and pharmaceutical arts, exemplified by a recent Patent Trial and Appeals Board decision holding unpatentable genus claims to glass-making method based on this Federal Circuit precedent. But the bulk of this portion of the argument is focused on the expected effects on claims in the biotechnology and pharmaceutical fields, based on the nature of these inventions, the complexities of the claimed subject matter, and the triviality of changes that can be made. These vulnerabilities are exacerbated by the costs of bringing a patented product to market, the brief citing as an average almost $3 billion, where the possibilities of “free-rider” issues that are enhanced under the full scope enablement test undermine incentives to invest. In addition to these negative effects on progress, Amgen argues the situation under the full scope test also delays effective disclosure if undue efforts must be undertaken for “rote identification of permutations within an invention”; the time and effort to do so will delay disclosure in patent applications until “sufficient” species within a genus have been identified to satisfy the full scope test.
Finally, the brief applies the rubrics to the case at bar, which the brief argues establishes that the specification enabled preparation of the 26 exemplified antibodies as well as providing a roadmap to making other embodiments using routine methods known in the art, and that Respondents could not show any failure to make any antibody falling within the scope of the invalidated claims. Amgen directly addresses Respondents’ argument that their claims “preempt the future” (likely one of the more compelling arguments for Respondents’ position) by ignoring the availability of further, later-arising improvements on the invention by others, citing Seymour v. Osborne, 78 U.S. (11 Wall.) 516, 548 (1871). The two juries’ verdicts that Amgen’s claims had not been shown not to be enabled was supported by “[m]ountains of evidence” they argue, and this evidence was not countered by any evidence that the disclosed methods ever failed. The brief enumerates the disclosures in the patents’ specifications regarding the 26 exemplified antibodies, the “roadmap” teaching how to produce additional antibodies and reliance on well-established prior art methods for making monoclonal antibodies generally, and the ability to identify conservative amino acid substitutions to produce antibodies having variations in structure but retaining the functional properties of the claimed antibodies that gave them their therapeutic effectiveness. While emphasizing the lack of factual evidence contrary to the juries’ verdicts, the brief concludes by emphasizing the error of law Amgen asserts against the Federal Circuit’s decision in its use of the full scope test in the face of a jury verdict supported by substantial evidence, in urging the Supreme Court to reverse the adverse decision below.